Dr. Klukkert, what is the current situation regarding continuous tablet production?
As a general rule, continuous manufacturing is working its way into pharmaceutical and nutrition production. At some manufacturers, however, reservations remain as they associate high investment and conversion costs with continuous manufacturing.
Why is that?
Apart from validation, which is associated with increased costs and effort regardless of the type of product conversion, some particular reservations can be observed: base areas which are still often rather extensive and the height of continuous systems, the long lead times for construction and manufacture, as well as the device prices which tend to be high as these plants are very customerspecific.Such calculations can swiftly give rise to a negative business case which is why the next step is often not even tackled.
How do you deal with this?
We basically ask ourselves what plant design is necessary in order to produce a positive business case. Continuous manufacturing will only assert itself wide scale if these typical reservations are countered with offers which are easier to adapt and therefore economically attractive. That means leaner plant designs and a simplified process chain, insofar as formulations make this possible. We can offer an attractive overall solution here that combines maximum production efficiency with minimum complexity.
What plant design would you favor?
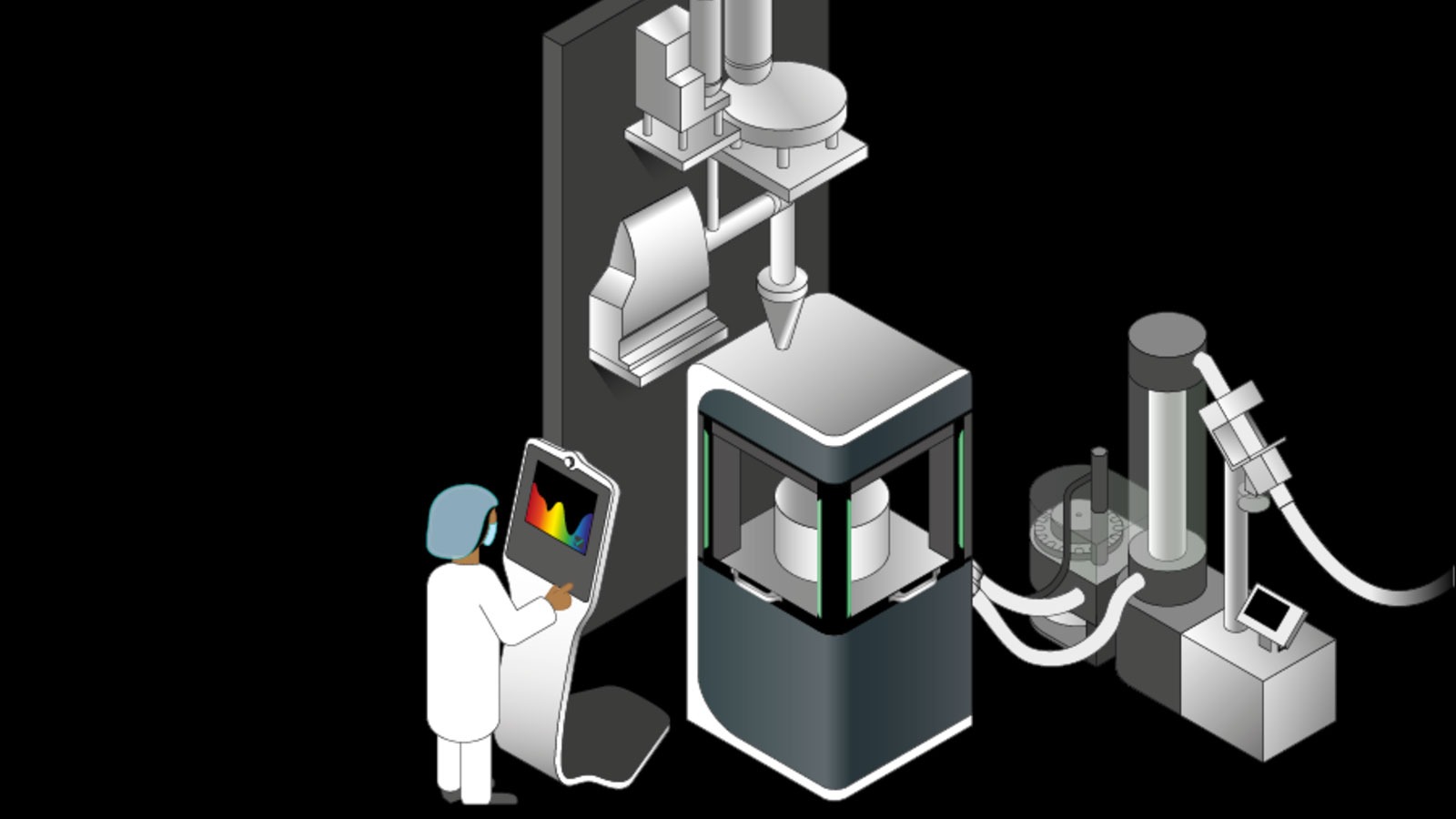
We specialize in direct compression. The powder is fed directly from the dosing mixer module into the tablet press without any additional granulation. This dispenses with several cost intensive production steps, such as roll compacting and grinding, which are required for dry granulation. As a result, this reduces the spatial requirements and steering complexity of the process. The low hold-up mass in the plant is a key factor for improved process steering and faster achievement of stable production conditions. A lean plant design also permits faster product changes and shorter cleaning times.

Sample overall design of a continuous direct compression line with material feed and dosing (upper level), mixing system and tablet press (lower level).
What role does the tablet press play in the process as a whole?
It is and will remain the heart piece of processing. We deploy a versatile FE55 rotary press for direct compression as it can be used to manufacture a wide range of different tablet types and formats in various configurations. The machine also features three instead of two compression rollers which permits longer dwell times at lower pressure. This gives rise to more gentle processing of raw materials and improves the versatility of the tableting process.
To what extent has direct compression asserted itself among users?
Initial pharmaceutical and nutrition producers have long been giving preference to direct compression over more complex production methods, accompanied by a corresponding focus on the raw materials. This has already resulted in several positive business cases which also take consideration of factors such as the personnel deployed and the number of cleaning cycles.
What do you recommend companies expressing interest?
The technological maturity of continuous production will improve fast. Innovative plant concepts are about to be launched which promise to offer a high potential for reducing complexity. Accordingly, users can count on new technological advances in continuous manufacturing. That is why our recommendation for companies expressing interest is to assess their respective potentials without delay.

Detailed view of the direct compression line: material feed and dosing station at the upper level.
»USERS CAN COUNT ON NEW TECHNOLOGICAL ADVANCES IN CONTINUOUS MANUFACTURING. AGAINST THIS BACKDROP, OUR RECOMMENDATION FOR COMPANIES EXPRESSING INTEREST IS TO ASSESS THEIR RESPECTIVE POTENTIALS WITHOUT DELAY.«
Dr. Marten Klukkert, Manager Technology Center und Pharmacist at Fette Compacting