Continuous production demands a new understanding of processes among the manufacturers of pharmaceuticals: the product in one process forms the direct base material for the next one. Processes and components which used to be separate are now merging to form a single line which, unlike the batch process, remains in operation without interruptions over longer periods of time. This change is also altering the requirements on process control: control systems are now necessary whose sensors can be reliably integrated into the continuous line and permanently record all of the relevant parameters.
Within the framework of tablet production, continuous direct compression has proven to be a feasibly attractive system design. Along with increased production efficiency, the process, which entails the sub-processes of dosing, mixing and tableting merging to form a single line, is particularly advantageous in terms of process reliability and product quality. As the powder is fed directly in a continuous flow from the mixer into the tablet press, quality-impairing factors can be significantly reduced, for example. This prevents vibrations during transport of intermediate products which could otherwise cause them to separate. Furthermore, a continuous direct compression line can be monitored particularly safely and efficiently thanks to the lean design of the system as a whole.
PAT for direct compression
The use of real-time quality control systems in continuous tableting safeguards both efficiency and product quality. By means of process analytical technology (PAT), the critical quality attributes (CQAs) of the material flow are constantly recorded, whereby highly-developed sensors should permit monitoring and control of all of the relevant process parameters. The specific process factors to be analyzed for consistently high product quality depend on the process steps of the continuous method.
The measurement technologies suitable for continuous lines can be roughly broken down into three groups: in-line, on-line and at-line technologies. In in-line and on-line processes, measurements are performed directly in the production flow which permits swift reactions in the event of deviations in quality and immediate control of the production process. The difference between the two variants lies in how the PAT system is integrated in the process: in the case of on-line technology, a separate sampling cycle is used while in-line technology measurements are carried out directly at the process flow.
In at-line technologies, samples are typically taken from the process. Ideally, the external test device can even be fully integrated in the production line, dispensing with the need for any additional interventions by operators. This means it is possible to carry out at-line analyses directly after production in the production area, enabling manufacturers to react swiftly to any process variations.
Near-infrared spectroscopy as a first choice
Near-infrared spectroscopy (NIRS) has proven to be the most efficient analytical method for direct compression. Its greatest advantage is the fact that the spectral range of 700 to 2,500 nanometers records numerous different active ingredients. The infrared rays penetrate deep into the tablet without causing any damage. As an in-line method, NIR spectroscopy permits faster quality controls of larger samples throughout the entire manufacturing process. This means it is eminently suitable for real-time use in high-performance machines (Figs. 1 and 2).
The same NIRS measurement also delivers information on both the chemical and certain physical attributes of a sample. The concentration of a substance in active preparations can be established just as reliably as other factors such as density and moisture content, for example. Consequently, NIRS has a major potential as a PAT tool in direct compression: it paves the way toward a better understanding of the process as a whole and attributes manufacturers full control over all essential production parameters.
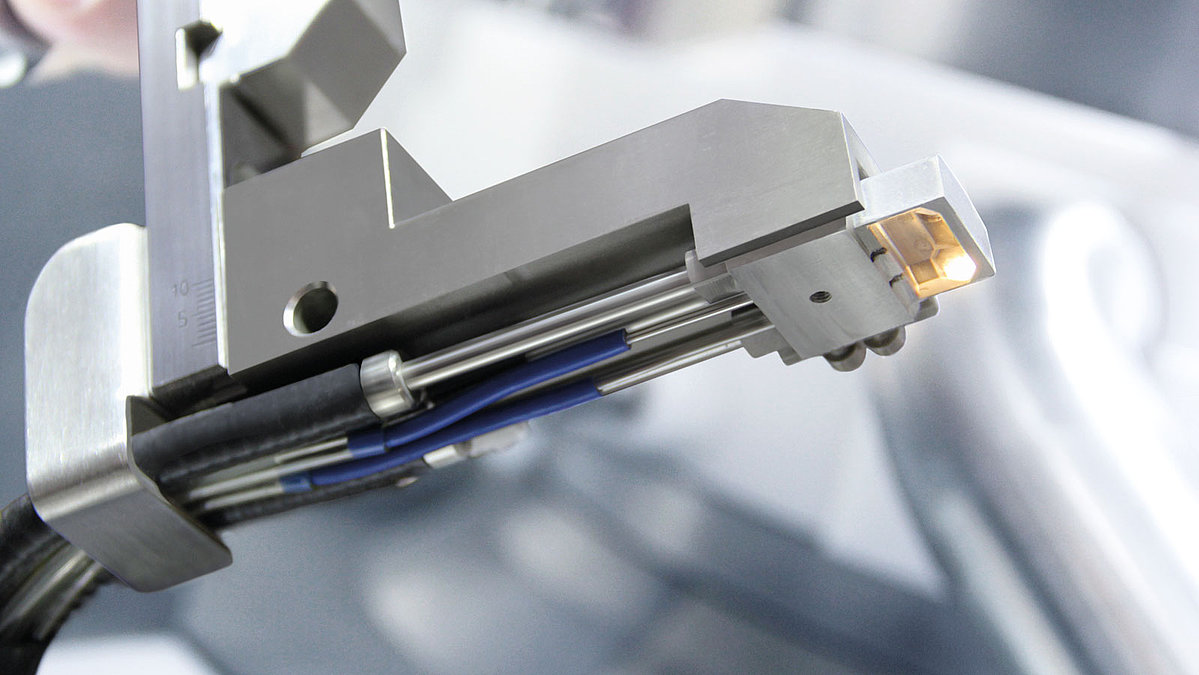
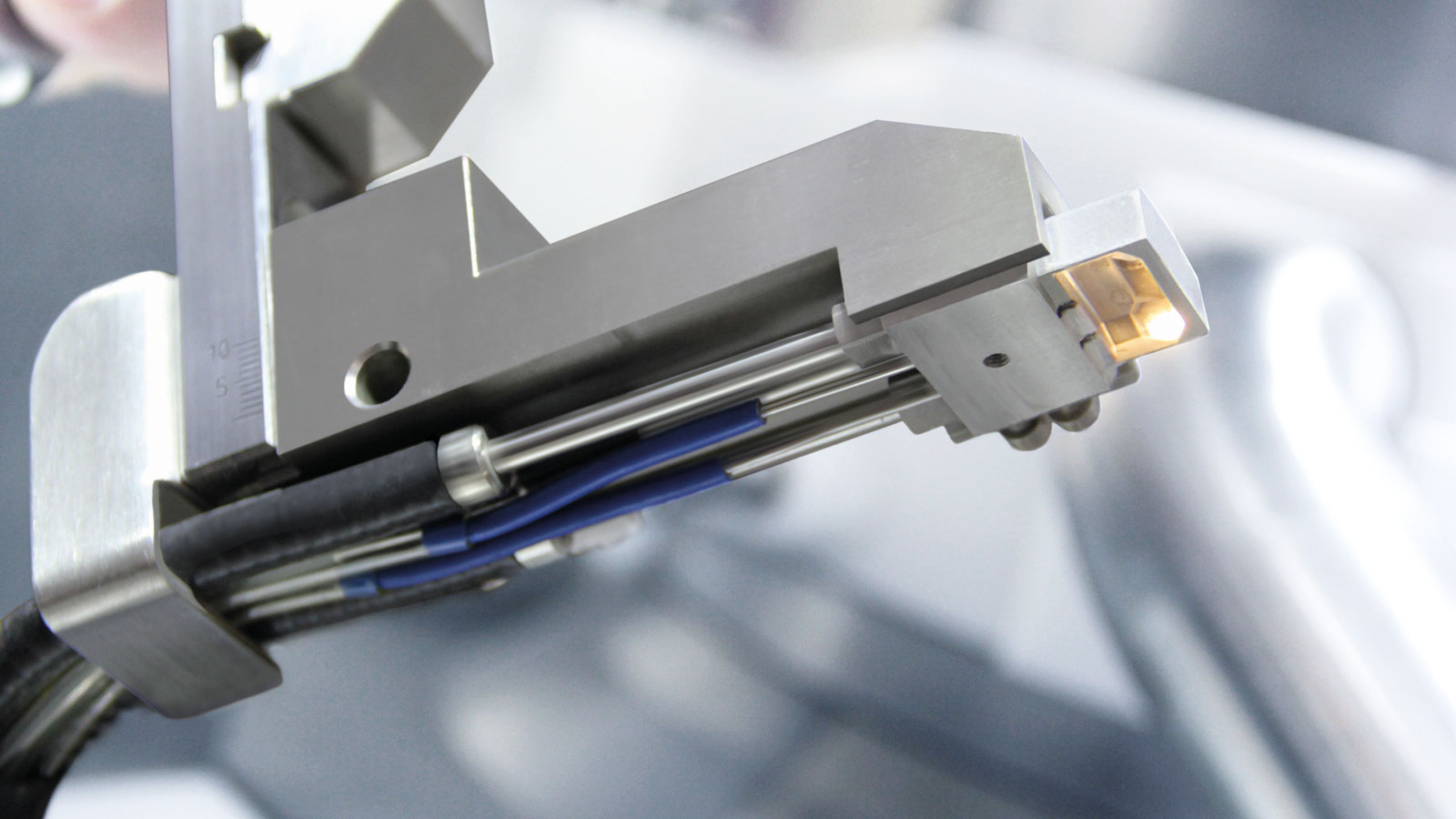 Fig. 1:NIRS measuring head for the tablet press in in-line mode
Fig. 1:NIRS measuring head for the tablet press in in-line mode


Fig. 2: In-line NIRS sensor at the filling station of a single rotary tablet press
Alternative processes for special cases
But near-infrared spectroscopy reaches its limits with an active ingredient content of less than one percent. In such cases, users can rely on Raman spectroscopy. Thanks to a strong laser (Fig. 3), it is capable of accurately determining even tiny concentrations of substance. Another advantage is the fact that it reacts less sensitively to process variations. Then again, it requires more experience in terms of operation in order to avoid over-layering by fluorescence, for example. In special cases, methods such as laser-induced fluorescence (LIF), UV, or terahertz spectroscopy can also be used.
The number and position of sensors used varies depending on the PAT technology and use case. Particularly in the area of R&D, a sensor is often used after the mixer before the powder reaches the tablet press. Sometimes a sensor is also deployed at the Fill-O-Matic. In-line measurement for checking tablets is carried out directly in the tablet press while at-line measurements are performed downstream in the Checkmaster (Fig. 4).


Fig. 3: Raman measuring head with a strong laser which records even the tiniest concentrations of active ingredient


Fig. 4: Sample Checkmaster for downstream analysis of active ingredients in at-line mode
Outlook
The various measurement methods have been subject to ongoing development for the best part of the last 20 years. However, PAT has not yet been able to assert itself on a broad scale in tablet production. Equipment is still being used which can only be handled safely and efficiently by an experienced specialist. Such technologies are often provided by third-party suppliers and are integrated in the production line using additional software which further increases the complexity of these systems. There remains a need for optimization toward more robust, easy-to-operate, and fully-integrated PAT systems. Suitable concepts are already being implemented.
To summarize: With the right process analytical technology, the direct compression method represents a quality-assuring and economically-feasible solution toward the goal of continuous pharmaceutical production. And in view of new plant concepts intimating further reductions in complexity in the near future, the analytical methods of near-infrared and Raman spectroscopy will play a decisive role. Users can look forward to new advances in technology in the area of continuous manufacturing which will define PAT and direct compression as an integral yet highly-flexible unit.
Please do not hesitate to contact us if you require more information and detailed advice. We look forward to receiving your email.
Contact:
Dr. Anna Novikova
Manager Application Center, Pharmacist at Fette Compacting
ANovikova@fette-compacting.com