
The FAT in Schwarzenbek thoroughly tested the performance of the 2090i WiP.
Dr. Reddy’s Laboratories relies on the highest safety standards in the processing of highly active ingredients through high containment and wash-in-place (WiP) technology. This Indian pharmaceutical company has collaborated with Fette Compacting to develop a suitable overall solution.
Dr. Reddy’s was established in southern India 40 years ago. Since then, the company has established itself worldwide with a broad portfolio comprising over 350 products in numerous therapeutic areas. These include, for example, drugs for gastrointestinal disorders, various types of cancer, cardiovascular diseases, infectious diseases, and pediatric diseases. Dr. Reddy’s mission, “Good Health Can’t Wait,” reflects its commitment to providing affordable generic medicines and innovative services to improve the lives of patients.
“As a manufacturer of highly active medicines, we are committed to using leading technologies to optimize our production processes while ensuring the highest safety standards,” explains Vivekanand Kamat, Site Head, FTO-SEZ Process Unit 2 at Dr. Reddy’s. “Especially for the production of tablets with active to highly active ingredients, we attach great importance to comprehensive containment. We need absolutely dust-tight equipment with closed material handling and transfer. For us, this also includes a wash-in-place function to thoroughly clean the press chamber before product changes, to protect our operators and avoid potential crosscontamination.”
Dr. Reddy’s is not alone in these requirements, as the pharmaceutical industry has been experiencing a clear trend toward highly active ingredients for years. Containment systems are therefore increasingly prevalent, as they reliably protect personnel from exposure and dispense with the need for using fully protective suits. Tailor-made containment packages offer safety, feasibility and user-friendliness in a single system.
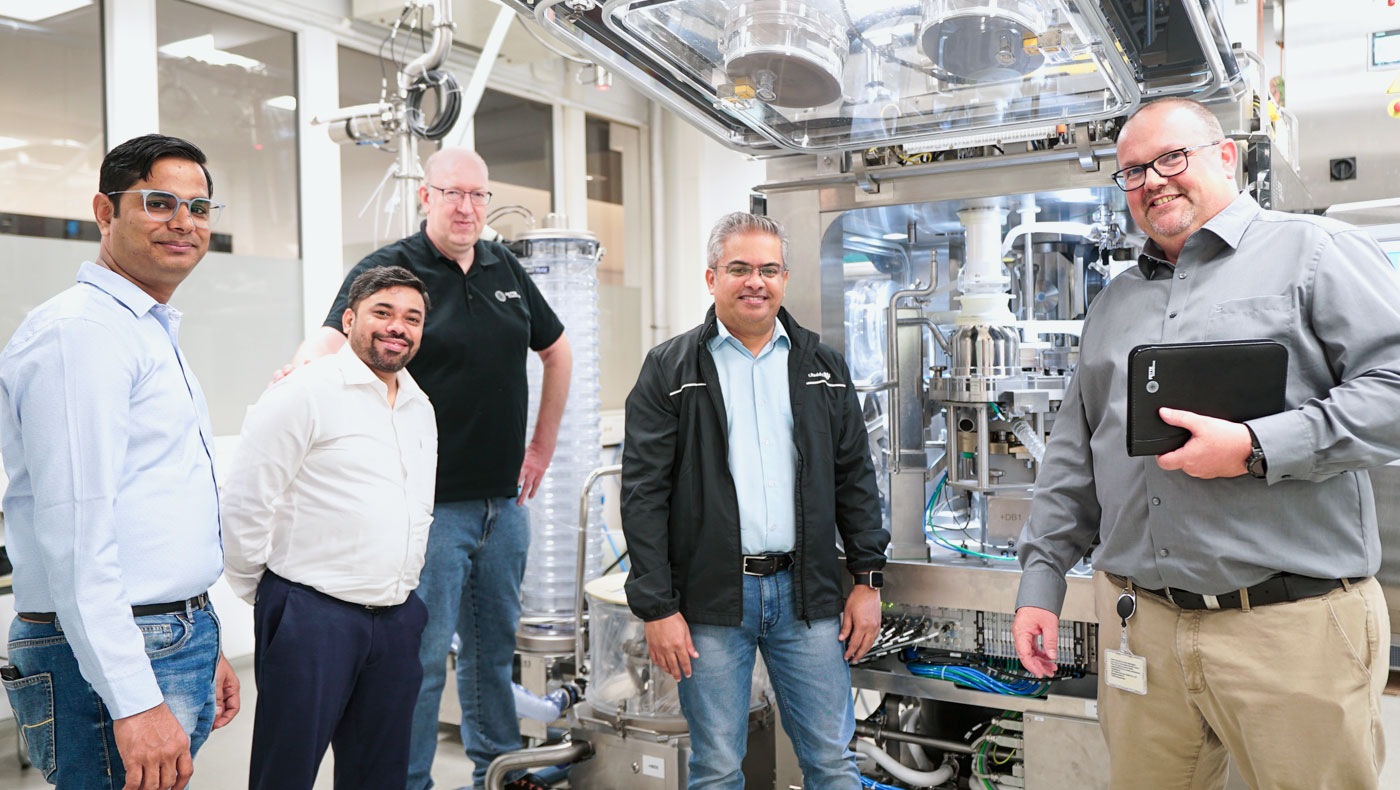
This partnership-based cooperation has led to a high level of satisfaction on both sides.
WiP and containment: the ideal combination
In its search for a suitable containment system, Dr. Reddy’s approached Fette Compacting and found the right solution in three tried-and-tested 2090i WiP tablet presses. They are characterized by an integrated wash-in-place system that ensures efficient and safe processing of highly active substances. The machines have a washable compression chamber with automatic preliminary cleaning. In addition, smooth stainless steel surfaces and tool-free access to the components that come into contact with the product facilitate manual cleaning and changeover work. This also enables significantly faster format changeovers.
The containment equipment provides complete control over dust emissions throughout the entire tableting process. An air management system continuously regulates the air flow. Glove ports allow maintenance or adjustment work to be carried out without breaching containment. In addition, hermetically sealed window flaps and rapid transfer ports (RTP) ensure the safe replacement of components.
“We expect output to remain constant within a tolerance of ten to a maximum of 15 percent during our production changeover,” notes Vivekanand Kamat. “Above all, we attach great importance to simple changeover systems that reduce the complexity of operation and minimize the risk of operating errors. The 2090i WiP has met and even exceeded these expectations right from the start.”

A washable containment system facilitates cleaning processes, protects the operators,and prevents cross-contamination.
From FAT to needs-based training
In summer 2024, the Factory Acceptance Test (FAT) of the 2090i WiP in Schwarzenbek marked an important step in the company’s partnership with Fette Compacting. “The FAT was well organized and the team had prepared carefully so that all tests were successfully completed on time,” claims Kamat with satisfaction. These positive experiences also underline the high adaptability of the containment and WiP technology to Dr. Reddy’s specific requirements. They confirm the commitment of both teams to meet the highest quality and safety standards.
Dr. Reddy’s also uses Fette Compacting’s extensive training and service offerings to support a smooth production start-up. “For example, we need audiovisual training aids that convey the process and the critical aspects of handling containment in standardized modules,” says Kamat. “The Fette Compacting team is very responsive to this need.” And Biswajit Panda, Manager-Production, FTO-SEZ Process Unit 2 at Dr. Reddy’s, adds: “We have the impression that we are in good hands here – with a partner who not only understands our technical requirements, but also proactively provides solutions for process optimization.”









